|
An investigation into the nature and danger of nuclear radiation
Developers:
Alan Darion
Kutztown Area School District
Kutztown, PA
Al Breaux
Analytical Research
Rohm and Haas Company
Grade Level:
Senior High School
Discipline:
Physics
Goals:
Upon completion of this unit, the student will
- Understand the random nature of nuclear decay and radiation
- Appreciate that risk assessment is a matter of probabilities
- Recognize the various ways to control exposure to radiation
- Deal with the problem of nuclear waste in a rational rather than emotional way
Objectives:
Upon completion of this unit, the student will be able to:
- Distinguish between ionizing and non-ionizing radiation
- Apply probability calculations to predict the outcome of random events
- Identify the three types of nuclear radiation and distinguish among their various penetrating powers and biological hazard
- Apply probability calculation to evaluate the risk benefit of a situation
- Determine the relationship between risk of exposure to radiation and time through an understanding of a substance's half-life
- Apply probability calculation to determine the nature of radioactive half-life
- Apply the principles of geometry to explain the inverse square law.
Background and Overview:
Students will be
given the task of evaluating the claims of an imaginary nuclear power
company that wants to dispose of its nuclear waste within their
community. They will first investigate the nature of nuclear radiation,
and then apply this to the problem of nuclear waste.
Students should
work in small lab groups. If enough radiation detectors are available,
then the class can proceed with their activities in the order given. If
the number of detectors is limited, then alternate paths through these
activities need to be considered.
Equipment:
- Radiation detectors
Geiger-Muller tube of sufficient sensitivity to detect alpha particles, and a scaler or computer and interface for counting.
Pasco's Introductory Computer G-M system is adequate if you have a PC
or Macintosh available (about $300). An older version for the Apple
II's that inputs into the game port will also do.
- Radioactive Sources:
Alpha, beta, and gamma sources which do not require an NRC license.
Available from Pasco (about $115)
ONE Iso-generator Kit for Ba-137m
Also available from PASCO (about $175)
- Dice
800 to 1000 will do, but electronic dice are faster
A computer program in LOGO plus for the Macintosh is included as an appendix and is on the disk
- Jelly Beans (about 50 per student)
- Electroscopes
- Sources of Non-ionizing radiation
Context
The local
power-company has proposed putting a high-level radioactive waste
disposal site in your area. Hoping to eliminate opposition from the
community, the company has offered it a very low rate for electric
power, and has scheduled a public meeting in the high school auditorium
to try to convince the public that they are in no danger from the site.
However, your
science teachers have gotten a copy of some of the claims they will
make at that meeting. They have decided that you will check them out in
your own laboratories first, and be prepared to present them at the
meeting.
Their assertion that nuclear waste can be disposed safely rests on the following claims:
Nuclear radiation is not necessarily harmful. The danger depends on
- Type of radiation
- Dose — the amount of radiation received
Dose may be controlled through
- Shielding
- Distance from the source
- Time
Your job is to
now investigate whether or not these claims are true, and to decide how
they would apply to the disposal of nuclear waste.
ACTIVITY 1: SOME LIKE IT HOT
Background
Radiation is
anything that radiates, that is, emerges from, and travels away from a
source, in a continuous way. Bullets may be said to radiate from a
machine gun although we usually do not describe it that way. Radiation
usually refers to tiny particles or bundles of energy that emerge
continuously from a source and travel through space away from it. Light
radiates away from a light bulb. In fact, light is a form of
electromagnetic radiation, about the only kind of radiation we can
actually see. Radio signals radiate from the antenna of the radio
station — these are another kind of electromagnetic radiation. Light —
electromagnetic radiation we can see, ultraviolet and other
electromagnetic radiation we cannot see, and various particles radiate
from the Sun.
All radiation is
not dangerous. A few years ago, there was a hypothesis that the kind of
electromagnetic radiation from power lines and even household
appliances might be dangerous, but this has not stood up to any
scientific research. The type of radiation that that is known to be
dangerous is described as ionizing radiation.
Ionizing
radiation is made of those types of particles (which from now on will
include "bundles" of electromagnetic energy which behave very much as
particles) that have enough energy to knock the electrons off the atoms
they pass through — turning them into electrically charged ions.
Exposure to this kind of radiation can harm the cells of our bodies,
either killing them outright, or altering them so that they can become
cancerous.
Activity
Charge an electroscope.
Touch it with your hand
WHAT HAPPENS TO THE LEAVES?


WHY?


Charge the electroscope again
AS LONG AS THE AIR SURROUNDING THE ELECTROSCOPE REMAINS AN INSULATOR, WHAT WILL HAPPEN TO THE LEAVES OF THAT ELECTROSCOPE?


Open the chamber with the leaves and bring a flame near them
WHAT HAPPENS TO THE LEAVES?


WHAT DOES THAT INDICATE HAPPENED IN THE AIR AROUND THE LEAVES AS A RESULT OF THE FLAME BEING NEAR?


Charge the electroscope again
Put the following
sources of radiation, one at a time, into the chamber with the leaves
for about five minutes (the flame itself consists of ions — it works
much faster).
- The "red" (alpha) nuclear source
- A light bulb
- An electric motor
WHICH SOURCE EMITS IONIZING RADIATION?

EXPLAIN WHY YOU THINK SO


ACTIVITY 2: WANT TO BET?
Background
The nucleus of an
atom is made of positively charged protons, and neutral neutrons. Since
like charges repel each other, and the strength of the force increases
as the square of the distance decreases, there is an extremely large
repelling force among the protons squeezed to within .0000000000001 cm
of each other. They are held within the nucleus by the nuclear force.
In large nuclei, the balance between these two forces is unstable, and
it is possible for a small part of the nucleus, called an alpha
particle consisting of two protons and two neutrons, to fly away,
leaving the decayed nucleus behind.
Neutrons are
inherently unstable; they can only exist for long periods of time if
joined with an appropriate number of protons in a nucleus. If there are
too many neutrons, then one of them may decay into a positive proton,
and a negative particle. This negatively charged beta particle (which
has the same characteristics as an electron) escapes from the nucleus,
leaving behind a nucleus that now has one less neutron, and one more
proton. This is another form of nuclear decay.
In either of
these processes, or if a nucleus has too much internal energy to be
stable, a "bundle" of energy may be released called a gamma ray. (These
are not really particles but rather extremely short wavelength
electromagnetic radiation, and so they are referred to as rays.
However, this distinction is very complicated and, for the purposes of
this discussion, not very important.) While the release of this
uncharged particle does not change the type of nucleus it came from, it
does allow its internal energy to reach a lower, more stable, state.
Materials made of
these unstable nuclei are said to be radioactive. In any of these
radioactive types of nuclear decay, it is impossible to predict when a
particular nucleus will actually decay. However we can determine the
probability that it will decay in some period of time. Let us see what
it is like when an event is controlled through its probability of
happening.
Activity
Consider a single die (one dice).
WHAT ARE THE ODDS THAT ON A SINGLE ROLL YOU WILL GET A 4? 
Roll it. WAS IT A 4? 
Now roll it again.
WHAT ARE THE ODDS THAT ON THIS ROLL YOU WILL GET A 4? 
Past events do
not effect future probability. As a result, we can never predict when a
particular roll will give a particular result. However, we can
determine what is the most likely outcome of many rolls.
WHAT IS THE MOST LIKELY NUMBER OF TIMES A 4 WILL COME UP IF THE DIE IS ROLLED 60 TIMES? 
WHAT IS THE MOST LIKELY NUMBER OF 4's YOU WILL GET IF YOU ROLL 60 DICE? 
Teacher Note:
Make sure you emphasize that the number expected is the number of dice
rolled times the probability of getting the desired outcome. |
Try it — either with real dice, or electronic ones.
HOW MANY 4's DID YOU GET? 
Try it again.
HOW MANY 4's DID YOU GET? 
Roll the 60 dice 8 more times, and record the number of 4's below.
       
Determine the
absolute value of the difference between the most likely outcome and
the actual outcome on each of these ten rolls.
        and the first two and the first two  
WHAT IS THE AVERAGE VALUE OF THESE DIFFERENCES?

WHAT PERCENT OF THE NUMBER OF DICE ROLLED EACH TIME (60) IS THIS?

Rolling 60 dice 10 times is the same as rolling 600 dice once.
WHAT IS THE MOST LIKELY TOTAL NUMBER OF 4's TO COME UP?

HOW MANY ACTUALLY CAME UP?

THE DIFFERENCE BETWEEN PREDICTED AND ACTUAL WAS

The difference
between these values should not be equal to the sum of the differences
calculated above, because those were the absolute values of the
differences.
WHAT PERCENT OF 600 IS THE DIFFERENCE BETWEEN THESE VALUES?

Summary
It is impossible
to predict exactly what will occur when events are controlled by
probability. For example, we cannot predict which individual dice will
come up 4. However we can always calculate what is the most likely
total outcome of a series of events if we know their probabilities. The
more events, the smaller the percentage difference between the most
likely outcome and the actual outcome.
If we have a
collection of unstable (radioactive) nuclei, it is impossible to
predict which nuclei will decay in a given amount of time. However, if
we know the probability that a nucleus will decay in a some amount of
time, we can predict the total number of that collection that are most
likely to decay in that time. Since a macroscopic sample of some
material would have at least somewhere around 1025 nuclei, we can
predict, with a very low percentage error, the total number that will
decay in that time. Of particular interest is the amount of time it
would take for the odds to be 50:50 that the nucleus of a material will
decay. This will be important in a later activity.
Activity 3: COUNT ON IT!
Background
The ionizing
property of nuclear radiation may be used to detect, and count, the
nuclear particles and rays in the surrounding environment. (For the
sake of simplicity in our language, discrete bundles of electromagnetic
energy referred to as rays may also be included when reference is made
to particles). The device used to detect the particles is called a
Geiger-Muller tube, (G-M tube) and when it is combined with a method of
counting those particles it is called a Geiger Counter.
The Geiger-Muller tube operates in the following manner

When the particle
passes through the "window" into the tube, it ionizes the gas inside.
(Some G-M tubes have no window so as not to block out the least
penetrating radiation, and others have thick windows that only allow
the most penetrating to be detected). This completes the circuit, and a
pulse of current passes through it. The counter, which is called a
scaler, or a computer programmed to act as a scaler, then counts the
number of such pulses.
Because of the
random nature of radioactive emission, you can never be "sure" that the
number of counts you have is the amount you should expect, i.e., the
most likely amount. However, the uncertainty can easily be calculated;
it is the square root of the total number of counts that have been
made. So
| if the count is 4 |
the uncertainty is 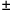 2 2 |
or 50% |
| if the count is 25 |
the uncertainty is 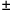 5 5 |
or 20% |
| if the count is 100 |
the uncertainty is 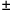 10 10 |
or 10% |
| if the count is 1,000 |
the uncertainty is 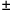 32 32 |
or 3.2% |
| if the count is 10,000 |
the uncertainty is 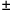 100 100 |
or 1% |
10,000 or more counts would be ideal, however you should try to get at least 1000 to be fairly certain of your results.
Activity
Set up your G-M
tube so it is held vertically above the source as shown below. Set the
value of d to be just big enough to allow five thicknesses of lead to
be inserted above the source.

Remove all sources and count the number of "background" particles that pass through in 5 one-minute time intervals.
 |
 |
 |
 |
 |
| minute 1 |
minute 2 |
minute 3 |
minute 4 |
minute 5 |
Calculate the average background radiation per minute 
(You could simply count for five minutes and divide this total by five, but your counter may not be set up to do this.)
Place the "red" source directly below the G-M tube with the "hole" facing up.
Count the number of particles to get into the tube in one minute with nothing between the source and the tube. Record in data.
Put successive
thicknesses of paper between the source and the tube. Record the number
of particles to get through in one minute for each thickness.
Remove the paper, and repeat for successive thicknesses of plastic, and then of lead.
Any time your
count has dropped to the background level, STOP, and begin this
procedure again with the "green", and then the "orange" source.
WHAT CAN YOU CONCLUDE ABOUT THE RADIATION COMING FROM THE THREE SOURCES? USE THE ABOVE DATA TO EXPLAIN YOUR ANSWER


WHAT DO YOU SUPPOSE HAPPENED WHEN THE FIRST PIECE OF PAPER WAS PUT OVER THE RED SOURCE?


WHAT MAY HAVE CAUSED THE GRADUAL DROP AS THE THICKNESSES OF PAPER OVER THE GREEN SOURCE WERE INCREASED?


WHAT DID THE PIECE OF PLASTIC DO TO THE RADIATION COMING FROM THE GREEN SOURCE?


WHAT EFFECT DO PAPER AND PLASTIC HAVE ON THE RADIATION COMING FROM THE ORANGE SOURCE?


WHAT MIGHT HAVE CAUSED THE GRADUAL DROP AS GREATER THICKNESSES OF LEAD WERE PUT OVER THE ORANGE SOURCE?


WHAT MIGHT YOU NEED TO STOP ALL THE RADIATION FROM THE ORANGE SOURCE FROM GETTING TO THE DETECTOR


THE LEAST PENETRATING RADIATION COMES FROM THE  SOURCE SOURCE
THE SECOND LEAST PENETRATING RADIATION COMES FROM THE  SOURCE SOURCE
THE MOST PENETRATING RADIATION COMES FROM THE  SOURCE SOURCE
Summary
The three types of particles are:
The alpha (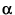 ) is doubly charged and extremely dense. ) is doubly charged and extremely dense.
The beta (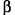 ) is singly charged and has several thousand times less mass than the alpha. ) is singly charged and has several thousand times less mass than the alpha.
The gamma (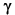 ) has no charge and is a "bundle" of pure energy with zero mass. ) has no charge and is a "bundle" of pure energy with zero mass.
WHICH DO YOU SUPPOSE IS THE MOST PENETRATING? 
WHY?

WHICH DO YOU SUPPOSE IS THE LEAST PENETRATING? 
WHY?

WHICH DO YOU SUPPOSE CAN DO THE MOST DAMAGE TO YOU? 
WHY?


UNDER WHAT CIRCUMSTANCES WOULD EXPOSURE TO THIS RADIATION BE MOST HARMFUL?


The fact that a
particle is "stopped" by some material means that it has interacted
with something in that material. For this reason, the very heavy,
highly charged alpha particles are the ones that are the least
penetrating. However, they are also the ones that could do the most
damage. If an alpha particle were to enter, or contact your body, it
will almost certainly interact with the cells of your body. This could
damage them immediately, causing immediate radiation damage, or alter
the genetic material of the cell so that it is more likely to become
cancerous in the future.
However, since
alpha particles are stopped by even a few inches of air, they are only
likely to interact with the cells of your body if they get on or inside
of you. This is only going to happen if you touch, inhale or eat a
source of alpha particles. Beta particles are slightly more penetrating
but they too pose a serious risk only if there is ingestion, inhalation
or direct physical contact with a source of these particles.
As a result, if
you are exposed to nuclear radiation from outside of your body, it will
most likely be to gamma rays. Because they have no charge or mass,
gamma radiation is the most difficult to stop. That also means a gamma
ray may pass completely through your body without interacting with any
of your body's cells. If that occurs, it has done you no harm. Once
again, we are dealing with probability, or the odds, that you will be
harmed by exposure to gamma radiation.
Current theory
holds that there is no absolutely safe amount of radiation that we can
tolerate. Any exposure carries with it some risk of harm. The amount of
risk depends on the amount of radiation, that is, the total number of
particles you are exposed to.
Before
investigating the factors other than shielding that affect the number
of particles you might be exposed to, let us examine this issue of risk
more carefully.
ACTIVITY 4: IS IT WORTH THE RISK?
Background
The current
approach to the biological hazard of radiation is that any exposure
carries with it a risk of damage. In other words, it is possible for a
single particle to interact with the DNA in one of your cells and
trigger the cascade of events which results in cancer. The more
particles one is exposed to, the greater the risk that such an adverse
event will actually happen.
However,
eliminating all risk is impossible. There is the constant source of
background radiation. There is risk in crossing the street, eating
bacon, getting too much exercise, getting too little exercise. In fact,
some research has found that you have the greatest risk of having a
heart attack when you get out of bed in the morning — usually on
Mondays. It is impossible to live without risk. The question is not
eliminating risk, but keeping it low enough so that the risks involved
in some activity are less than the benefits that may be received.
This is not a
scientific question. The value of the benefit, and the hazard of the
risk, are really personal values. However, the following activity might
give you a sense of the balance that you might strike, and a sense of
how probability governs your decisions.
Activity
You have two assortments of jellybeans — the "sweet" and the "spice".
Wash your hands.
Pick out fifty of your favorite flavors and put them in the jar.
Now pick out a flavor you absolutely hate.
Replace one of the jellybeans in the jar with one of these and shake the jar to mix them up.
WHAT ARE THE ODDS YOU WILL GET ONE YOU LIKE? 
WHAT ARE THE ODDS YOU WILL GET ONE YOU HATE? 
Close your eyes, and pick one bean out of the jar.
ARE YOU WILLING TO EAT IT? 
If not STOP here
If yes, then you MUST eat it whatever it is.
WHAT KIND WAS IT? 
If it was a "bad" one you may replace it and repeat the trial before moving to the next one.
Replace another one of the "good" beans with one of the "bad".
WHAT ARE THE ODDS YOU WILL GET ONE YOU LIKE? 
WHAT ARE THE ODDS YOU WILL GET ONE YOU HATE? 
Close your eyes, and pick one bean out of the jar.
ARE YOU WILLING TO EAT IT? 
If not STOP here
If yes, then you MUST eat it whatever it is.
WHAT KIND WAS IT? 
If it was a "bad" one you may replace it and repeat the trial before moving to the next one.
Now replace three more (a total of five)
WHAT ARE THE ODDS YOU WILL GET ONE YOU LIKE? 
WHAT ARE THE ODDS YOU WILL GET ONE YOU HATE? 
Close your eyes, and pick one bean out of the jar.
ARE YOU WILLING TO EAT IT? 
If not STOP here
If yes, then you MUST eat it whatever it is.
WHAT KIND WAS IT? 
If it was a "bad" one you may replace it and repeat the trial before moving to the next one.
Continue to
replace five at a time until the BENEFIT of getting one you like is not
worth the RISK of getting one you hate, and you decide to stop.
WHEN YOU QUIT:
WHAT WERE THE ODDS YOU WOULD GET ONE YOU LIKE? 
WHAT WERE THE ODDS YOU WOULD GET ONE YOU HATE? 
Questions for Discussion
- Why did you decide to stop?
- Why do you suppose other people chose to stop at other times?
- Did you decide to stop after getting a bad one?
- Suppose the "bad" one did not just taste bad, but made you ill. Would you have stopped at the same place?
- Suppose the
"bad" one did not just taste bad, but was a deadly poison, and was
mixed in with a million "good" ones. How many would you eat from those
million?
- The following
have been estimated to increase your risk of dying by one in a million
chances (Adapted from DOE Radiation Worker Training, based on work by
B.L Cohen)
- Smoking 1.4 cigarettes (lung cancer)
- Eating 40 tablespoons of peanut butter
- Spending 2 days in New York City (air pollution)
- Driving 40 miles in a car (accident)
- Flying 2500 miles in a jet (accident)
- Canoeing for 6 minutes
- Receiving 10 mrem of radiation (cancer)
Which of these risks have you been taking? Which ones will you continue.
- Do you think
the willingness to take these risks of those who have had personal
experience with cancer (either themselves or someone close to them)
might be different from that of those who have not?
ACTIVITY 5: THAT'S ONLY THE HALF OF IT
Background
Teacher Note:
Check the maximum counting rate your detector can handle and still be
accurate. With the Pasco SE — 7981 for the Apple II's do not have an
initial counting rate of over 80 per sec. (4800 per Min). You only want
to "milk" the iso-generator of 1 ml. |
Radioactive
materials do not emit radiation forever. Remember that the release of a
particle changes the nucleus it came from. Let us consider the simplest
situation; the one in which the new nucleus is not radioactive. Once
every nucleus in a macroscopic amount of this material has decayed by
emitting a particle, the material would stop being radioactive.
You will be given
a sample of an isotope of Barium, Ba 137m. Remember, while you cannot
predict which nuclei will decay in any particular amount of time (let's
use one minute), you can predict that the number of nuclei that will
decay during that time. It will be the total number of radioactive
nuclei you have times the probability that any one of them will decay
in that time.
Activity A
Place the
radioactive material given to you by your teacher under the G-M tube as
quickly as you can (but no so fast you spill any).
Record the number
of particles emitted every 30 seconds for ten minutes, and then
calculate the rate (counts per minute) during each of these intervals.
Graph the rate vs. time
WHAT HAPPENS TO THE RATE AT WHICH PARTICLES ARE EMITTED AS TIME GOES ON?

THE RATE OF
EMISSION DEPENDS ON THE NUMBER OF RADIOACTIVE NUCLEI AND THE
PROBABILITY ONE WILL EMIT A PARTICLE IN ONE MINUTE. WHICH OF THESE
FACTORS WILL CHANGE AS TIME GOES ON?


CONSIDER THE RATE
AT WHICH PARTICLES WERE EMITTED DURING THE FIRST HALF-MINUTE INTERVAL.
USE THE GRAPH TO DETERMINE HOW LONG IT TOOK FOR THIS RATE TO DROP TO
HALF THIS NUMBER. LET US CALL THIS TIME INTERVAL 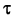 . .
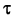 = = 
OF THE ORIGINAL NUMBER OF NUCLEI, WHAT DOES THIS TELL US IS THE FRACTION THAT WERE STILL RADIOACTIVE AFTER THE INTERVAL 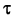 ? ? 
AND SO, WHAT FRACTION WAS NO LONGER RADIOACTIVE? 
PREDICT WHAT THE RATE WILL BE AFTER AN ADDITIONAL TIME INTERVAL 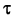 . . 
LOOK AT THE GRAPH. WHAT WAS THE ACTUAL RATE? 
ARE THESE VALUES SIGNIICANTLY DIFFERENT? 
CONSIDER THE RATE AT ANY TIME. WHAT HAPPENS TO THAT RATE AFTER AN INTERVAL 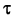 FROM THAT TIME? FROM THAT TIME?


Summary Activity A Background Activity B
The rate drops in half because the number of radioactive nuclei drops in half during the time interval 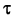 . For this reason . For this reason 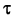 is referred to as the half-life of the substance. It is the time it
takes for half of all the nuclei of a radioactive substance that you
have at the moment to emit their particle and decay into different
nuclei.
is referred to as the half-life of the substance. It is the time it
takes for half of all the nuclei of a radioactive substance that you
have at the moment to emit their particle and decay into different
nuclei.
There is another
way to calculate the half-life of a material. It is the time it takes
for the probability of decay to become 1 in 2 (50:50). This time does
not depend on the number of nuclei decaying. It is a constant
characteristic of the material. Let us use our dice (real or
electronic) to see this. Since the probability of an even number coming
up are 1 in 2, let us say that any die that comes up even represents a
nucleus that has emitted a particle and decayed. Each roll of the dice
would then represent the time it takes for the odds of decay to become
50:50.
Activity B
Start with 800
dice and begin rolling them. (If these are real dice splitting them up
among the entire class might be a good idea).
Count the number
of even dice — these represent the number that decayed and so would
also give you the number of particles emitted during this roll.
Calculate the number of odd dice — these represent the number of nuclei
that did not decay and are therefore still radioactive.
Remove them — they represent nuclei that are no longer radioactive, and roll the remaining ones.
Continue for 5 rolls and record your results on the worksheet.
WHAT WOULD YOU HAVE PRDICTED THESE VALUES TO BE?

WERE THE ACTUAL
VALUES SIGNIFICANTLY DIFFERENT THAN THE PREDICTED VALUES? REMEMBER, THE
PERCENTAGE UNCERTAINTY FOR THESE NUMBERS IS FAIRLY HIGH.

The number of
"radioactive" nuclei, and therefore the number of radioactive particles
emitted during each roll, should have dropped by close to one half on
each roll. How does the time represented by each roll (which is the
time in which the probability for decay becomes 1 in 2) compare to the
half-life 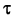 ? ?


Questions for Discussion
- If you have a collection of radioactive nuclei, will they be most radioactive at first, or after a few half-lives of time?
- You have the same number of nuclei of two different substances - one with a half-life of two weeks, Substance A. The other, Substance B,
has a half-life of two years. The number of nuclei in both substances
is such that after 10 half-lives, any radiation emitted is
indistinguishable from background
- Which will be the most radioactive during the first month?
- Which will be the most radioactive after a year?
- If you are constantly exposed to both sources, which one will give you the greatest dose of radiation in one year?
- If you are constantly exposed to both sources, which one will give you the greatest dose of radiation in twenty years?
- If both substances emitted the same type of radiation at the same energy, which would you say is the most dangerous. Why?
- If you have a substance with an extremely long half-life, let us say several hundreds of years
- Would it remain radioactive for a long time or a short time?
- Under what conditions would a sample of such a substance present a high risk to those exposed to it?
Summary
The situation in
this investigation is the simplest we can have. When these radioactive
nuclei decay, the resulting nuclei are not radioactive. Often, this is
not the case. These daughter nuclei may well be radioactive, emitting
different particles and having a different half-life. There may be a
cascade of decays before a stable arrangement is finally reached. As a
result it is possible for a single radioactive nucleus to be the
ultimate source of several particles — maybe up to ten or so.
ACTIVITY 6: DON'T BE SQUARE
Background
If you happen to
be walking along one day and an evil executive from the power company
rolls a ball of nuclear waste at your feet (a possibility you may lose
sleep worrying about), what do you suppose you should do?
A. Pick it up to bring to the authorities.
B. Run away
C. Call your best friend over to have a look.
That's right! B is correct.
You already know
that alpha particles do not go very far. A little bit of air will stop
them, and almost any kind of barrier will stop beta particles. But what
about gamma rays.
Activity
Set up your equipment as it was in Activity 3, shown below.

Teacher Note:
You may have to use some trial and error until you find the correct calibration point for the distance your detector. |
The source should
be the orange, gamma source. The distance d should be measured to the
window in the G-M tube, not to the bottom of the protective sleeve.
Find the counts
per minute at each of the distances d shown in the Activity 6
worksheet. You may have to count for several minutes to get
statistically useful data at the larger distances. If so, make sure all
data points are normalized to the same amount of time.
Calculate the square of each of the distances and record in the table above.
FROM YOUR DATA,
TRY TO DETEREMINE THE FRACTION OF THE COUNTS AT 2 CM YOU WERE GETTING
AT 4 CM. REMEMBER THE UNCERTAINTY OF THESE VALUES.
CONTINUE WITH THE OTHER DISANCES INDICATED
AT 4 CM THERE WERE  THE COUNTS AS AT 2 CM THE COUNTS AS AT 2 CM
AT 8 CM THERE WERE  THE COUNTS AS AT 2 CM THE COUNTS AS AT 2 CM
AT 16 CM THERE WERE  THE COUNTS AS AT 2 CM THE COUNTS AS AT 2 CM
DO YOU SEE THE RELATIONSHIP BETWEEN DISTANCE AND RATE OF EXPOSURE?
TRY GRAPHING THE COUNTS DETECTED VS. THE SQUARE OF THE DISTANCE, AND THEN AGAINST THE INVERSE OF THE SQUARE OF THE DISTANCE.
THE INTESITY OF RADIATION (NUMBER OF COUNTS) AT A PARTICULAR LOCATION DECREASES AS THE  OF THE DISTANCE FROM THE SOURCE INCREASES. OF THE DISTANCE FROM THE SOURCE INCREASES.
Summary
We call this the
inverse square law. Anything that radiates from a source uniformly in
all directions obeys this law. Doubling the distance (multiplying it by
2) drops the intensity by one-fourth (1 divided by 2 squared).
Increasing the distance 10 times (multiplying it by 10) makes the
intensity 100 times less (dividing it by 10 squared). It is really a
matter of geometry, not science.
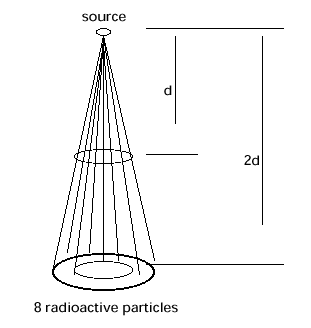
All eight
particles travel through the cone, and all eight pass through the ends
of the cone at distance d and at 2d from the source at the apex of the
cone. However the radius of the circle at the end of the cone at 2d is
twice as big as that at d (similar triangles), and so its area is four
times bigger since area goes as r squared. As a result, the number of
particles passing through an area at 2d that is the same as the area of
the circle at the end of the cone at d, is four times less — in this
case only two. If the source is not limited to a point, the effect of
distance may not follow this pattern exactly for small distances.
ACTIVITY 7: SPREAD IT AROUND
Background
In nuclear
explosions, within nuclear reactors and particle accelerators,
radioactive particles are brought to very high energy levels. These
high energy particles can interact with stable nuclei and change them
into the unstable nuclei of radioactive isotopes. There is an
underlying fear that all radiation, even the lower energy particles
that are not being accelerated, can do the same thing. In other words
the quality of being radioactive can be caught, like a contagious
illness. Let us see if this can happen.
Activity
Take three coins and check them with a rate meter (or your computer set to METER)
IS THE RATE GREATER THAN BACKGROUND? 
Put one on each of the sources and leave them there over night.
Check them the next day with a rate meter.
IS THE RATE GREATER THAN BACKGROUND NOW? 
FOR WHICH ONE(S) 
While exposure to radiation may not make something radioactive, contact is another matter.
Use a rate meter to see if there is any radiation coming from a paper towel.
IS THE RATE GREATER THAN BACKGROUND? 
Make sure you have gloves on or have your teacher do this!
Let a drop from
the iso-generator (from the half-life activity) fall on the paper
towel. Quickly, check the rate coming from the wet spot on the towel.
IS THE RATE GREATER THAN BACKGROUND? 
Summary
While
radioactivity cannot be spread like a virus or bacteria through
contagion, it can be spread through contamination. That is, the
radioactive material can mix with or adhere to other substances. It can
then spread throughout the environment through the natural migration of
those substances.
Questions for Discussion
- What are some examples of ways this can happen?
- Which of these do you consider the most likely?
Why?
- Which of these do you consider the most dangerous?
Why?
ACTIVITY 8: CONCLUSION
Background
You should now be in a position to evaluate the claims of the power-company. These were:
Nuclear radiation is not necessarily harmful. The danger depends on
- Type of radiation
- Dose — the amount of radiation received
Dose may be controlled through
- Shielding
- Distance from the source
- Time
You need to
consider the validity of these claims as they apply to nuclear waster.
In order to evaluate the risk of having a high-level waste disposal
sight in your community, you will need to deal with a unit of measure
called the rem. This is the unit used to express the amount of damage
radiation can do to your body. It is a found by multiplying the amount
of radiation you are exposed to by the biological effect that that
particular radiation might have. For example, alpha particles are
between 10 and 20 times more damaging than an equal number of gamma
rays.
Activity
Do an Internet
search on the risk of exposure to nuclear radiation, or read over the
three articles included in the Appendix. One of these articles is
obviously anti nuclear, one seems to be from the nuclear power
industry, and another from the science department of a university —
although they may all claim academic affiliation. You should be aware
of these biases when you evaluate and use their content.
Prepare a written
statement of the position you would take at the town meeting to discuss
the power-company's proposal. Make sure this statement includes your
own evaluation of the risks involved. This evaluation must be supported
with the evidence of your investigation. It may include information
from your Internet search, but the validity of this information must be
evaluated in terms of the observations and conclusions you made in your
activities.
Suggested Web Sites
http://www.umich.edu/~radinfo/introduction/risk.htm
http://whatisnuclear.com/articles/waste.html
Suggested Search Words
radiation + risk
nuclear waste
hormesis
Appendix One: Electronic Dice
TO POUT :S :E
TELL 0 HT
TELL :S
SETTFONT "GENEVA
SETTSTYLE [1 12]
IF :S > :E [ODDEVEN STOP STOP]
MAKE "A ( STRING `THE NUMBER OF ` :S `'s = ` THING WORD :S "S )
TELL :S STAMPTEXT :A 200 29
POUT :S + 1 :E
END
TO SETCOUNT :S :E
MAKE WORD :S "S 0
IF :S > :E [STOP]
TELL :S PU HT SETX -100 SETY 100 - 25 * :S
SETCOUNT :S + 1 :E
END
TO DICE :N
HT
CT
SETWFONT "LOGO [GENEVA 14]
SETCOUNT 1 6
DICE.DO 1 :N
POUT 1 6
AGAIN
DICE :NUMB
END
TO DIE
MAKE "XX ( 1 + RANDOM 6 )
( PRINT1 :XX ` ` )
END
TO NUMBER
PR `How many dice do you want to roll?`
MAKE "NUMB READWORD
IF NOT NUMBER? :NUMB [NUMBER STOP]
END
TO ODDEVEN
MAKE "ODD :1S + :3S + :5S
MAKE "EVEN :2S + :4S + :6S
TELL [7 8]
SETTFONT "GENEVA
SETTSTYLE [1 12]
TELL 7 PU
SETXY -100 -75
STAMPTEXT STRING `EVEN = ` :EVEN 150 30
TELL 8 PU
SETXY -100 -100
STAMPTEXT STRING `ODD = ` :ODD 150 30
END
TO MANYDICE.DO :S :E
IF :S > :E [STOP]
MANYDIE
CHECKIT 1 6
MANYDICE.DO :S + 1 :E
END
TO MANYDICE :N
HT
CT
SETWFONT "LOGO [GENEVA 14]
SETCOUNT 1 6
MANYDICE.DO 1 :N
POUT 1 6
AGAIN
MANYDICE :NUMB
END
TO MANYDIE
MAKE "XX ( 1 + RANDOM 6 )
END
TO SETUP
CT DRAW HT
SETWPOS "LOGO [5 100]
SETWSIZE "LOGO [400 300]
SETWPOS "TURTLE [400 100]
SETWSIZE "TURTLE [300 300]
END
TO VISIBLE
PR `Do you want to see the numbers on the dice? `
PR `It goes faster if you do not.`
PR []
PR `Press Y or N and then return`
MAKE "AAA READWORD
IF :AAA = "Y [DDD DICE :NUMB STOP]
IF :AAA = "N [DDD MANYDICE :NUMB STOP]
PR []
PR `Y or N only please!`
WAIT 10
VISIBLE
END
TO AGAIN
PR []
PR `Do you want to roll this number of dice again`
PR `Press Y or N and then return`
MAKE "BBB READWORD
IF :BBB = "Y [STOP]
IF :BBB = "N [DOIT STOP]
PR `Y or N only please!`
WAIT 10
AGAIN
END
TO DICE.DO :S :E
IF :S > :E [STOP]
DIE
CHECKIT 1 6
IF ( REMAINDER :S 15 ) = 0 [PR []] [PRINT1 ` `]
DICE.DO :S + 1 :E
END
TO DOIT
SETUP
NUMBER
VISIBLE
END
TO CHECKIT :S :E
IF :S > :E [PR "????? TOPLEVEL STOP]
IF :XX = :S [MAKE WORD :S "S 1 + THING WORD :S "S STOP]
CHECKIT :S + 1 :E
END
TO DDD
CT
TELL 0
SETTFONT "GENEVA
SETTSTYLE [5 12]
PU HT
SETXY -100 100
STAMPTEXT ( STRING `ROLLING ` :NUMB ` DICE` ) 150 30
END
MAKE "A `THE NUMBER OF 6's = 58`
MAKE "AAA "N
MAKE "XX 6
MAKE "BBB "N
MAKE "1S 71
MAKE "ODD 208
MAKE "3S 65
MAKE "2S 46
MAKE "EVEN 168
MAKE "5S 72
MAKE "4S 64
MAKE "7S 0
MAKE "6S 58
MAKE "NUMB 376
This experiment is courtesy of 
|
|



