Definition
An electrolytic capacitor is a type of capacitor that uses an ionic conducting liquid as one of its plates with a larger capacitance per unit volume than other types.
Basics
An electrolytic capacitor is a type of capacitor
typically with a larger capacitance per unit volume than other types,
making them valuable in relatively high-current and low-frequency
electrical circuits.
This is especially the case in power-supply filters, where they store
charge needed to moderate output voltage and current fluctuations, in rectifier output, and especially in the absence of rechargeable batteries that can provide similar low-frequency current capacity. They are also widely used as coupling capacitors in circuits where AC should be conducted but DC should not; the large value of the capacitance allows them to pass very low frequencies.
The electrolytic capacitor was invented in 1921 by Julius Edgar Lilienfeld.
It was largely responsible for the development of mains-powered radio
receivers, since it permitted the filtering of the 50-60 hertz power
supplied to residences, after it was rectified to power the radio
tubes. This was not practical without the small volume and low cost of
electrolytic capacitors.
Construction
Aluminium electrolytic capacitors are constructed from two conducting aluminium foils, one of which is coated with an insulating oxide layer, and a paper spacer soaked in electrolyte. The foil insulated by the oxide layer is the anode while the liquid electrolyte and the second foil act as cathode.
This stack is then rolled up, fitted with pin connectors and placed in
a cylindrical aluminium casing. The two most popular geometries are
axial leads coming from the center of each circular face of the
cylinder, or two radial leads or lugs on one of the circular faces.
Both of these are shown in the picture.
Polarity
In aluminum electrolytic capacitors, the layer of insulating
aluminum oxide on the surface of the aluminum plate acts as the
dielectric, and it is the thinness of this layer that allows for a
relatively high capacitance in a small volume. The aluminum oxide layer can withstand an electric field strength of the order of 109 volts per meter. The combination of high capacitance and high voltage result in high energy density.
Unlike most capacitors, electrolytic capacitors have a voltage
polarity requirement. The correct polarity is indicated on the
packaging by a stripe with minus signs
and possibly arrowheads, denoting the adjacent terminal that should be
more negative than the other. This is necessary because a reverse-bias
voltage will destroy the center layer of dielectric material via
electrochemical reduction (see Redox reactions). Without the dielectric material the capacitor will short circuit,
and if the short circuit current is excessive, then the electrolyte
will heat up and either leak or cause the capacitor to explode.
Modern capacitors have a safety valve,
typically either a scored section of the can, or a specially designed
end seal to vent the hot gas/liquid, but ruptures can still be
dramatic. Electrolytics can withstand a reverse bias for a short period
of time, but they will conduct significant current and not act as a
very good capacitor. Most will survive with no reverse DC bias or with
only AC voltage, but circuits should be designed so that there is not a
constant reverse bias for any significant amount of time. A constant
forward bias is preferable, and will increase the life of the capacitor.
Electrolyte
The electrolyte is usually boric acid or sodium borate in aqueous solution together with various sugars or ethylene glycol
which are added to retard evaporation. Care should be taken to avoid
ingestion of or eye contact with the electrolyte, and any areas of the
body where skin contact has occurred should be washed in good time. It
is important to follow safe working practice and to use appropriate
protective equipment, notably gloves and safety glasses, when working
with the electrolyte. Some very old tantalum electrolytics, often
called "Wet-slug", contain the more hazardous sulfuric acid, however
most of these are no longer in service due to corrosion.
Electrical behavior of electrolytics
A common modeling circuit for an electrolytic capacitor has the following schematic:
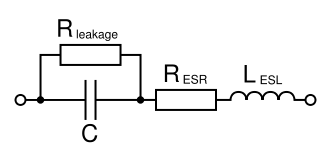
where Rleakage is the leakage resistance, RESR is the equivalent series resistance, LESL the equivalent series inductance (L being the conventional symbol for inductance).
RESR must be as small as possible since it determines the loss power when the capacitor is used to smooth voltage. Loss power scales quadratically with the ripple current flowing through and linearly with RESR. Low ESR capacitors are imperative for high efficiencies in power supplies.
It should be pointed out that this is only a simple model and does
not include all the effects associated with real electrolytic
capacitors.
Since the electrolytes evaporate, design life is most often rated in hours at a set temperature.
For example, typically as 2000 hours at 105 degrees Celsius (which is
the highest working temperature). Design life doubles for each 10
degrees lower, reaching 15 years at 45 degrees.
Capacitance
The capacitance
value of any capacitor is a measure of the amount of electric charge
stored per unit of potential difference between the plates. The basic
unit of capacitance is a farad, however this unit has been too large for general use until the invention of the Double-layer capacitor, so microfarad and picofarad are more commonly used.
Many conditions determine a capacitor's value, such as the thickness of the dielectric and the plate area. In the manufacturing process, electrolytic capacitors are made to conform to a set of preferred numbers. By multiplying these base numbers by a power of ten, any practical capacitor value can be achieved, which is suitable for most applications.
A standardized set of capacitor base numbers was devised so
that the value of any modern electrolytic capacitor could be derived
from multiplying one of the modern conventional base numbers 1.0, 1.5, 2.2, 3.3, 4.7 or 6.8
by a power of ten. Therefore, it is common to find capacitors with
values of 10, 15, 22, 33, 47, 68, 100, 220, and so on. Using this
method, values ranging from 0.1 to 4700 are common in most
applications. Values are generally in microfarads (µF).
Most electrolytic capacitors have a tolerance range of
20 %, meaning that the manufacturer guarantees that the actual
value of the capacitor lies within 20 % of its labeled value.
Selection of the preferred series ensures that any capacitor can be
sold as a standard value, within the tolerance.
Variants
Unlike capacitors that use a bulk dielectric made from an
intrinsically insulating material, the dielectric in electrolytic
capacitors depends on the formation and maintenance of a microscopic
metal oxide layer. Compared to bulk dielectric capacitors, this very
thin dielectric allows for much more capacitance in the same unit
volume, but maintaining the integrity of the dielectric usually
requires the steady application of the correct polarity of direct current
else the oxide layer will break down and rupture, causing the capacitor
to fail. In addition, electrolytic capacitors generally use an internal
wet chemistry and they will eventually fail as the water within the
capacitor evaporates.
Electrolytic capacitance values are not as tightly-specified as with
bulk dielectric capacitors. Especially with aluminum electrolytics, it
is quite common to see an electrolytic capacitor specified as having a
"guaranteed minimum value" and no upper bound on its value. For most
purposes (such as power supply filtering and signal coupling), this
type of specification is acceptable.
As with bulk dielectric capacitors, electrolytic capacitors come in several varieties:
- Aluminum electrolytic capacitor:
compact but lossy, these are available in the range of <1 µF to
1 F with working voltages up to several hundred volts DC. The
dielectric is a thin layer of aluminum oxide. They contain corrosive
liquid and can burst if the device is connected backwards. The
electrolyte will tend to dry out in the absence of a sufficient
rejuvenating voltage, and eventually the capacitor will fail. Bipolar
electrolytics contain two capacitors connected in series opposition and
are used for coupling AC signals. Bad frequency and temperature
characteristics make them unsuited for high-frequency applications.
Typical ESL values are a few nH.
- Tantalum:
compact, low-voltage devices up to several hundred µF, these have a
lower energy density and are more accurate than aluminum electrolytics.
Tantalum capacitors are also polarized because of their dissimilar
electrodes. The cathode electrode is formed of sintered tantalum grains, with the dielectric electrochemically formed as a thin layer of oxide.
The thin layer of oxide and high surface area of the porous sintered
material gives this type a very high capacitance per unit volume. The
anode electrode is formed of a chemically deposited semi-conductive
layer of manganese dioxide, which is then connected to an external wire lead. A development of this type replaces the manganese dioxide with a conductive plastic polymer (polypyrrole) that reduces internal resistance and eliminates a self-ignition failure.
- Compared to aluminum electrolytics, tantalum capacitors have very stable capacitance, little DC leakage, and very low impedance
at low frequencies. However, unlike aluminum electrolytics, they are
intolerant of voltage spikes and are destroyed (often exploding
violently) if connected in the circuit backwards or exposed to spikes
above their voltage rating.
- Tantalum capacitors are more expensive than aluminum-based
capacitors and generally only usable at low voltage, but because of
their higher capacitance per unit volume and lower impedance at high frequencies, they are popular in miniature applications such as cellular telephones.
- Electrolytic double-layer capacitors (EDLCs), also known as supercapacitors or ultracapacitors,
have very high capacitance values but low voltage ratings. They use a
molecule-thin layer of dielectric in combination with porous materials
that have a high internal surface, such as carbon nanotubes or aerogels.
As the energy stored is inversely proportional to the thickness of the
dielectric and proportional to the surface, these capacitors have an
extremely high energy density. The electrodes are made of activated carbon, which has a high surface area per unit volume, further increasing the capacitor's energy density.
Individual EDLCs can have capacitances of hundreds or even thousands of
farads. For example, the Korean company NessCap offers units up to
5000 farads (5 kF) at 2.7 V, useful for electric
vehicles and solar energy applications. Smaller units (in the
0.1 F – 10 F range) are frequently used instead of
(or in addition to) batteries to supply standby power to memory
circuits and clocks.
The electrodes for EDLCS could also be made by transition metal oxides, eg. RuO2, IrO2, NiO, etc. Electrodes made by metal oxides store the charges by two mechanism: double layer effect, the same with active carbon, and pseudocapacitance, which can store more energy than double layer effects.
- Aerogel capacitors, using carbon aerogel
to attain immense electrode surface area, which allows them to attain
huge values, up to thousands of farads. EDLCs can be used as
replacements for batteries in applications where a high discharge current is required, e.g. in electric vehicles.
They can also be recharged hundreds of thousands of times, unlike
conventional batteries which last for only a few hundred or thousand
recharge cycles. However, unlike batteries, capacitor voltage is
directly proportional to the total energy remaining, so a DC to DC converter must be used to maintain voltage and avoid waste.
Source: Wikipedia (All text is available under the terms of the GNU Free Documentation License and Creative Commons Attribution-ShareAlike License.)
|



