|
This experiment is courtesy of 
|
|

|

|
Straw Chromatography
|
|
Developers:
|

|
Cecelia Oberholzer
Little Flower High School
Philadelphia, PA
|
Dr. Peter Cartier
Sheryl Deley
Rohm and Haas Company
Spring House, PA
|
|
|

|
|
|
|
|
Grade
Levels:
|

|
High School
|
|
|
|
Discipline:
|

|
Chemistry
|
|
|
|
Goals:
|
|
- To acquaint students with the principles of liquid
chromatography.
- To construct a liquid chromatography column.
- To investigate the effects of different solvents and
solvent concentrations in the separation process.
|
|
|
|
Specific
Objectives:
|

|
To obtain separations of both synthetic dyes and natural
plant pigments.
|
|
|
|
Background:
|

|
Chromatography is a method of separation mixture of two
or more substances by distribution between two phases, one
of which is stationary and one of which is moving.
The type of chromatography used depends on the nature of
the two phases. Column chromatography, also called liquid
chromatography or LC is a solid-liquid phase partitioning
method. Small columns are packed with stationary phase
material called adsorbent. This material may be any solid
that does not dissolve in the liquid phase. Silica gel,
SiO2 X H2O, is commonly used as the
stationary phase.
The adsorbent (stationary phase) is continuously washed
by a solvent (liquid phase) passing through the column. The
mixture to be separated is introduced into the top of the
column. The components of the mixture initially adsorb onto
the silica particles and are then washed down by the solvent
at differing rates, depending on their attraction, first to
the adsorbent and then to the solvent. The components of the
mixture form moving bands, and each band usually contains a
single component.
Good separation of mixture components (solutes) depends
on the kind of adsorbent and the solvent system. In general,
nonpolar compounds pass through the column faster than polar
compounds. If the adsorbent has equal affinity for the
solute materials, the components will not move down the
column. If the solvent is too polar, all the solutes may
wash down the column with no separation.
|
|
|
|
|
|
|
|
Materials:
|

|
|
Clear drinking straws
|
Glass wool
|
|
Silica gel (70-230 mesh)
|
Amberchrom� Ion Exchange Resin (Rohm and
Haas)
|
|
Ethanol
|
Water
|
|
Acetic acid, 0.1M
|
Wash bottle
|
|
Ring stand
|
Clamp holder
|
|
Extension clamp micro jaws
|
Dropper pipet
|
|
Microtip dropper pipet
|
Red food color (Durkee)
|
|
Washable black ink
|
Green and blue food colors (McCormick)
|
|
Beet juice
|
100-ml beakers
|
|
|
|
|
|

|
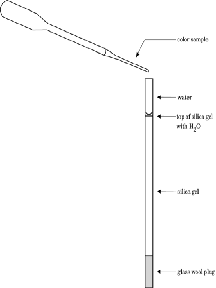
A. Silica Gel Column
- Wet a small piece of glass wool and plug the
bottom of the straw.
- Clamp the straw vertically to the ring
stand.
- Place a 100-ml beaker under the straw.
- In another beaker, add 20ml of water to a
small amount of silica gel to form a slurry.
- Draw some slurry into the dropper pipet.
- With a finger on the bottom of the straw,
holding the glass wool in place, add silica gel
slurry to the top of the straw from the dropper
pipet.
- Fill the straw with slurry to about three
centimeters from the top of the straw.
- Add water to the top of the straw column
using a wash bottle.
- Observe the water draining through the
slurry.
- Using a microtip dropper, add a drop of
green food color to the top of the silica gel in
the column. Let the color go into the gel.
- Add water to the top of the column,
continuing until the separation is complete.
- Collect the effluent from each band of color
in a separate beaker.
|
|
B. Acid Silica Gel Column
- Pack column using Procedure A, steps 1 though 9
(above).
- Rinse with 0.1M acetic acid (CH3COOH).
- Rinse with one volume of water.
- Using a microtip dropper, add one drop of beet juice.
- Add water to the top of the column, continuing until
the separation is complete.
- Collect the effluent from each band of color in a
separate beaker.
C. Amberchrom Resin Column
- Wet a small piece of glass wool and plug the bottom
of the straw.
- Clamp the straw vertically to the ring stand.
- Place a 100-ml beaker under the straw.
- Pack the straw column with Amberchrom� resin.
- Rinse the column with two volume of 30% ethanol.
- Add one drop of Durkee red food color to the top of
the resin.
- Add 30% ethanol until the color separation is
complete.
|
|
|
|
Exercise:
|
|
Amberchrom� Column Experiment
- Pack column using Procedure C, steps 1 through 4
(above).
- Rinse the column with two volumes of 10% ethanol.
- Add a drop of Durkee red food color.
- Continue the separation.
a. What happens to the color bands?
b. What is the effect of solvent concentration on the
separation?
- Repeat the procedure to separate washable black ink
using 10% ethanol as the solvent.
a. Is the separation of color complete?
b. If color remains on the column, what should be done to
remove the color?
|
|
|
|
Questions:
|
|
- What is chromatography?
- What two factors are important for good separation of
mixtures?
- Name one polar solvent and one nonpolar solvent.
- Name two adsorbent materials used in chromatography.
- What are the advantages of liquid chromatography
techniques?
- Investigate these other types of chromatography:
Paper chromatography
Thin layer chromatography
Gas chromatography
How do these types differ from liquid
chromatography?
- Investigate commercial uses of chromatography.
|
|
|
|
References:
|

|
- Krstulovic, A.M., Reversed-Phase High-Performance
Liquid Chromatography, John Wiley and Sons, New York,
1982.
- Thompson, S., Chemtrek, Allyn and Bacon,
Boston, 1990.
|
|
|
This experiment is courtesy of 
|
|



